|
GC665XD
 OverReaction
OverReactionType: Mystery | Size: Micro  | Difficulty:
| Difficulty: 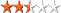 | Terrain:
| Terrain: 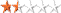 By: MrPolleyClass @ | Hide Date: 11/01/2015 | Status: Available Country: United States | State: Colorado Coordinates: N40° 22.837 W105° 03.586 | Last updated: 08/30/2019 | Fav points: 0
Add cache to watch list Log your visit Picture Gallery
GC663YY Knot So Fast (3.46 kms NE) GC9H2F5 Monday Morning Meetup 205 (4.61 kms N) GC412HZ Far an Taine ‘n Abhainn, ‘s Ann as Niò a (19.79 kms NW) GC7ZR9V Flying Saucers and Cone Heads (20.30 kms N) GC40J9A C M #4 (24.59 kms N) |
Driving Directions
5 Logs: |






